Primary Colors: The Art of Chemistry
Lecture Connections
| Keywords | Topics | Description |
| solute, solvent | Properties of matter | A solvent dissolves a solute. Food coloring is a solute. Water is a solvent. |
| concentration | Molarity; solutions | The quantity of solute present in a quantity of solvent. A UV-vis spectrometer is capable of measuring solution concentration. |
| electronic structure | Atomic structure | In this lab, a UV-vis spectrometer measures the interaction of ultraviolet and visible light with solutions of food coloring. |
| spectrum | Electromagnetic spectrum | A UV-vis spectrum is produced by the absorption of light. |
| color | Electromagnetic spectrum | In order for a compound to have color, it must absorb visible light. |
The relationship between primary colors and secondary colors can be shown using a color wheel, a tool commonly used in art to describe how colors are prepared. The Primary Colors are Red, Yellow, and Blue. Primary colors cannot be made by mixing other colors. However, mixing primary colors produces secondary colors. The Secondary Colors, Orange, Green, and Violet, are prepared by mixing primary colors; red & yellow make orange, yellow and blue make green, blue and red make violet. On a color wheel, the secondary colors lie between the two primary colors that make the secondary color.
A Color Wheel: Secondary Colors Lie Between the Two Primary Colors

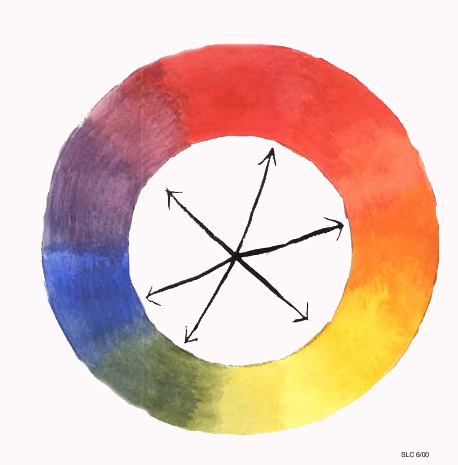
Another classification of colors is complimentary color. Complimentary colors consist of one primary color and one secondary color. Complimentary colors are opposite each other on the color wheel (arrows), and when mixed together make a shade of brown.
Complimentary Colors: Opposite Each Other on the Color Wheel

Mixing the three primary colors, or three secondary colors produces a gray color.
Red & Blue & Yellow ---> Gray
Orange & Green & Violet ---> Gray
UV-Visible Spectroscopy
A UV-vis Spectrophotometer is a research instrument used to gather information about a chemical sample. It exposes a chemical solution to the ultraviolet and visible region of the electromagnetic spectrum. Depending on the type of chemical, a certain amount of the light gets absorbed by the chemical which causes electrons to be promoted from one energy level to another. The amount of light that reaches the detector is then recorded as a spectrum as shown in Figure 1.
The UV-vis spectrum shown in Figure 1 plots the wavelength on the X-axis and the absorbance on the Y-axis. In this spectrum, green food coloring shows two distinct absorbance maxima; one peak is at approximately 420 nm with an absorbance of 0.45 while the other is at 640 NM with an absorbance of 0.80. The shape of the spectrum and the wavelength of maximum absorbance are characteristic of the chemical compound. The absorbance of compounds is also directly related to the concentration of the sample.
In order for a compound to have color, it must absorb visible light. The visible range of the electromagnetic spectrum comprises the wavelengths from about 400 to 800 NM Humans are especially sensitive to wavelengths around 655 NM (red). Our eyes see the wavelengths of light that are reflected by a compound. Compounds with color absorb light in the visible region but we see the complementary color that is reflected most completely. A white shirt, for example, "transmits" the visible light and feels cooler than a black shirt that "absorbs" the visible light. Similarly, a blue shirt absorbs light in the red region and transmits light in the blue region.
Most simple organic compounds absorb light in the ultraviolet region below 400 NM These compounds do not have colors that we see because our eyes only record light in the visible range of the spectrum from 400 to 800 NM According to Planck’s Law, E = HC/ l , the wavelength of light, l , is inversely proportional to the energy transition (E) of the electrons. Certain wavelengths of light cause electrons to jump to a higher energy level. In order for a compound to absorb at higher (i.e. visible) wavelengths, the energy of the electron transition must be lower. In practical terms, this transition requires large organic molecules with several aromatic rings.
In order for a compound to have color, it must absorb visible light. The visible range of the electromagnetic spectrum comprises the wavelengths from about 400 to 800 NM Humans are especially sensitive to wavelengths around 655 NM (red). Our eyes see the wavelengths of light that are reflected by a compound. Compounds with color absorb light in the visible region but we see the complementary color that is reflected most completely. A white shirt, for example, "transmits" the visible light and feels cooler than a black shirt that "absorbs" the visible light. Similarly, a blue shirt absorbs light in the red region and transmits light in the blue region.
Most simple organic compounds absorb light in the ultraviolet region below 400 NM These compounds do not have colors that we see because our eyes only record light in the visible range of the spectrum from 400 to 800 NM According to Planck’s Law, E = HC/ l , the wavelength of light, l , is inversely proportional to the energy transition (E) of the electrons. Certain wavelengths of light cause electrons to jump to a higher energy level. In order for a compound to absorb at higher (i.e. visible) wavelengths, the energy of the electron transition must be lower. In practical terms, this transition requires large organic molecules with several aromatic rings.
Spectroscopy is the study of how light interacts with matter, and certain regions of light in the electromagnetic spectrum have important environmental implications. For instance, molecules in the skin undergo certain chemical reactions when exposed to light in the ultraviolet region of the electromagnetic spectrum. One of these reactions is the synthesis of melanin. Since too much ultraviolet light overwhelms this reaction mechanism and causes permanent changes to the skin's structure, sunscreens can absorb some of this ultraviolet light. The figure below shows the structure and UV-visible spectrum of the active ingredient in sunscreen.
Spectrum of the Active Ingredient in Sunscreen
Notice how the sunscreen molecule absorbs light in the ultraviolet region of the electromagnetic spectrum.