Lecture Connections
FD&C Dyes in Gatorade
| Keyword |
General Concept |
Explanation |
|
Solute,solvent |
Classifying matter |
The distilled water is the solvent, the dye is the solute |
|
Concentration |
Molarity; solutions |
A UV-Vis spectrometer is capable of measuring concentration |
|
Electronic structure |
Electron structure, electromagnetic spectrum |
In this lab, a UV-Vis spectrometer measures the interaction of ultraviolet and visible light with solutions of food coloring |
|
Spectrum |
Electromagnetic spectrum; absorbance spectrum |
A UV-Vis spectrum is produced by the absorbance of light |
|
Color |
Electromagnetic spectrum |
For a compound to have color, it must absorb energy in the visible light range |
|
Dyes |
Organic molecules |
Food dyes are used in many foods to enhance color. |
Color in Organic Compounds
In order for a compound have color, it must absorb light in the region of the electromagnetic spectrum to which our eyes are sensitive. The visible range of the electromagnetic spectrum comprises the wavelengths from about 400 to 800 nm. Humans are especially sensitive to wavelengths around 655 nm (red). Our eyes see the wavelengths of light in the visible range that are reflected by a colored compound. Colored compounds absorb light in the visible region but we see the complementary color that is reflected most completely. A white shirt, for example, "transmits" the visible light and feels cooler than a black shirt that "absorbs" the visible light. Similarly, a blue shirt absorbs light in the red region and transmits light in the blue region.
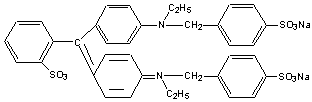
Most simple organic (carbon-containing) compounds absorb light in the ultraviolet region below 400 NM These compounds do not have colors that we see because our eyes record light only in the visible range of the spectrum (400 to 800 NM). According to Planck�s Law, E = hc/ l , the wavelength of light, l , is inversely proportional to the energy transition (E) of the electrons. Certain wavelengths of light cause electrons to jump to a higher energy level. In order for a compound to absorb at higher (i.e. visible) wavelengths, the energy of the electron transition must be lower. In practical terms, this transition requires large organic molecules with several aromatic rings. See Figure 2 of FD&C blue #1.
Figure 2: FD&C blue #1

These large organic molecules are thus used as synthetic dyes in foods. In this lab, you will compare the spectra from red, blue, and yellow food dyes to a spectrum of your assigned Gatorade sample. If your Gatorade sample contains a certain food dye, it will have an absorbance peak at the same wavelength and with the same shape as the spectrum from your known food dyes.
UV-Visible Spectroscopy
A UV-vis Spectrophotometer is a research instrument used to gather information about a chemical sample. It exposes a chemical solution to the ultraviolet and visible region of the electromagnetic spectrum. Depending on the type of chemical, a certain amount of the light gets absorbed by the chemical which causes electrons to be promoted from one energy level to another. The amount of light that reaches the detector is then recorded as a spectrum as shown in Figure 1.
The UV-vis spectrum shown in Figure 1 plots the wavelength on the X-axis and the absorbance on the Y-axis. In this spectrum, green food coloring shows two distinct absorbance maxima; one peak is at approximately 420 nm with an absorbance of 0.45 while the other is at 640 nm with an absorbance of 0.80. The shape of the spectrum and the wavelength of maximum absorbance are characteristic of the chemical compound. The absorbance of compounds is also directly related to the concentration of the sample.
Gatorade IngredientsExample Problem
The maximum absorbance is the absorbance reading (y-axis) for the top of the peak. The wavelength for the maximum absorbance peak is read from the x-axis. At the top of the peak (508 nm), the absorbance is .948.