Sunscreen Analysis
Teacher Version
Introduction:
There are a variety of excellent websites that include background data on UV
light and the role of sunscreens in skin protection. These should be available
as part of the InterChemNet package. Alternately, they can easily be located
with online searches. The science behind sunscreens is incredibly interesting
and also somewhat taken for granted.
Purpose:
Beer's law demonstrates the direct correlation, under ideal circumstances, between
the concentration of a solution and its absorbance at a given wavelength. Some
of the more complicated derivative math and history of this equation can be
found on the Vermont chemistry website.
Chemicals:
Because the students will need sunscreens from home, it is important to request
earlier that they bring them in from home. Greater variety among brands and
SPF values will yield more interesting results (especially when trying some
of the extra credit extension activities).
Note that isopropyl alcohol is being used as a solvent, not water. Although relatively safe (it can be purchased at the drugstore), isopropyl alcohol (a.k.a. isopropanol and 2-propanol) is flammable and can cause irritation. Consult an MSDS sheet for safety information and disposal instructions. Students should be made aware of the warnings and necessary precautions.
Materials:
The mixing plates aren't a necessity, but they greatly improve the efficiency
of the solution process. If plastic cuvettes are used they should be cleaned
immediately after the students finish their scans otherwise they may be ruined.
An alternate approach to making the dilutions that doesn't include the burettes
is described below.
Procedure:
(1) A balance that is sensitive to the milligram position is crucial for this
lab. Make sure that students understand the limits on the amount of sunscreen
needed. Examples of too much and too little sunscreen are shown below.

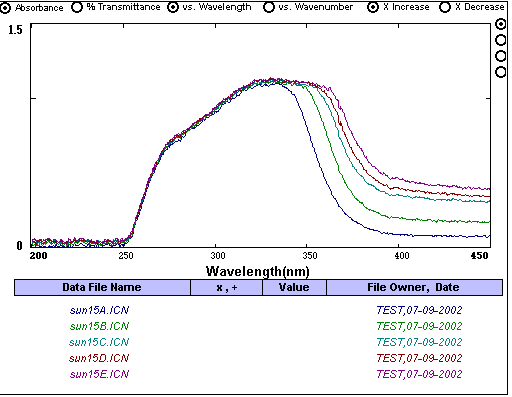
^This is an example of too much sunscreen having been used. The UV blockers are so highly concentrated that the solution is holding as much of them as it can. Even the diluted solutions are at maximum saturation, so the absorbance in the UV range doesn't change. This invalidates the Beer's Law relationship.
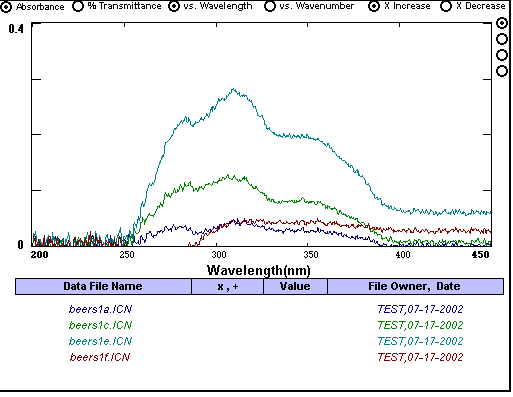
^This is an example of too little sunscreen being used (or any highly diluted spectrophotometer scan). Minor changes in concentration and other error inducing factors are very influential in highly diluted solutions. These result in a lack of uniformity among the curves and their peaks. Little information can be drawn from these scans.
If students are having difficulty getting their sunscreens to dissolve, it is best that they start over with a more soluble sunscreen. If there is a large amount of debris in the solution, the results will be flawed.
Proper use of the magnetic stirrers is important to prevent splattering of the isopropyl alcohol.
Students should be familiar with volumetric procedures before attempting this lab. Being able to read a fluid level (i.e. looking at the bottom of the meniscus) is assumed in the lab's write up.
Science Note: Combining the sunscreens with water first places them in colloidal suspension. The result is an opaque mixture that cannot be scanned (hence, water is not a proper solvent in this lab). However, the colloidal sunscreen has a greater surface area and therefore dissolves more readily in a less polar solvent. This extra step prevents large amounts of sunscreen debris from remaining outside of solution.
(2) The dilution procedure is not overly complicated for students that are familiar with using burettes. If students are inexperienced at this, it is recommended that someone in the lab group practice releasing controlled amounts of water or solution from the spouts before starting the actual lab procedure. If the burette procedure is too complicated, there is an alternate approach:
Instead of using burettes and beaks students can simply combine their sunscreen stock and alcohol in the cuvettes themselves using droppers. For example, using 20 drops of solution and 10 drops of alcohol is equivalent to the 4 parts 2 parts dilution. It is important that the volume of the cuvettes is known before hand, as this will shape the amount of drops used to fill them in the dilutions. For purposes of the math section, it will also be necessary to convert drops into milliliters at the end. Students can figure out the conversion themselves or the number can be supplied for them.
(3) Scanning the cuvettes
Refer to the library of common mistakes that occur during scanning
for help with this section. It is important that the machine be blanked with
isopropyl alcohol and not water during this lab.
Students should be familiarized with the function of the spectrophotometer and
analysis software before starting the lab.
Analysis:
The math here is spelled out fairly clearly. Students need
only to plug their numbers into the supplied formula (extra steps will be required
if using the dropper approach described previously). The Beer's law plot is
best done in Excel or another graphical analysis program. This assumes that
one of the students in each lab group is familiar with the software before starting.
If not, there are only five data points, so doing the graph by hand will not
be terribly difficult.
Maine State Learning Results (MSLR): This lab encourages skills listed in the following performance indicators under content area for secondary scores:
E. The Structure of Matter:
J. Inquiry and Problem Solving
Students will apply inquiry and problem-solving approaches in science and technology. Students will be able to:
Make accurate observations using appropriate tools and units of measure.
Verify , evaluate, and use results in a purposeful way. This includes analyzing and interpreting data, making predictions based on observed patterns, testing solutions against the original problem conditions, and formulating additional questions.
Demonstrate the ability to use scientific inquiry and technological method with short-term and long-term investigations, recognizing that a there is more than one way to solve a problem. Demonstrate knowledge of different strategies.
K. Scientific Reasoning
Students will learn to formulate and justify ideas and to make informed decisions. Students will be able to:
3. Develop generalizations based on observations
4. Determine when there is a need to revise studies in order to improve their validity trough better sampling, controls, or data analysis techniques.
L. Communication
Students will communicate effectively in the application of science and technology. Students will be able to:
3. Make and use appropriate symbols, pictures, diagrams, scale drawings, and models to represent and simplify real-life situations and to solve problems.
4. Employ graphs, tables, and maps in making arguments and drawing conclusions.
7. Use computers to organize data, generate models, and do research for problem solving.