Analyzing Sunscreens
Learning Goals:
1. To practice important solvation and dilution techniques.
2. To make connections between absorbance of EM radiation and
chemical structure.
3. To explore Beer's law and its importance in spectroscopy.
Abstract:
When one thinks of the energy delivered to the Earth from the
sun, it is common to consider only the light that is visible to the human eye.
However, the sun's rays consist of a wide variety of wavelengths in the electromagnetic
spectrum. One of the most troubling types of electromagnetic radiation is ultraviolet
(or UV). UV rays are not visible to the human eye, and are characterized by
wavelengths shorter than those of visible light (between 1 and 400 nm). UV rays
are responsible for giving skin a tanned color when one sits in the sun. However,
these same rays also induce premature aging of the skin, and-In some cases-the
genetic mutations that eventually give rise to skin cancer.
In this lab you will apply the techniques and mathematics of
spectroscopy to an investigation of UV-blocking ability of sunscreens. By analyzing
the absorbance in the UV range of a sunscreen solution, you will be able to
make predictions about its chemical composition and strength. You will also
apply your data to an application of Beer's Law: an important relationship between
concentration and absorbance.
VIEW LECTURE CONNECTIONS
| Materials |
Chemicals |
· 3 Beakers (at least 100 ml)
· 2 Burettes
· Electronic Balance
· Stirring Plate
· Stirring wand
· Burette holding stand
· Magnetic Stirrer
· Funnel
· 6 or more cuvettes
· 100 ml Volumetric Flask
· Spectrophotometer
|
· Water
· 150
ml Isopropyl Alcohol (a.k.a.
isopropanol or 2- propanol)
· Your favorite sunscreen
|
Procedure:
(1) Making the Sunscreen Solution
Begin by placing a beaker on the electronic balance. Tear
the scale so that it reads 0.000 g (this will subtract the beaker's mass from
subsequent measurements).
Now, very carefully, add a drop of sunscreen to the beaker.
Your goal should be to deposit between 0.025 g and 0.075 g of sunscreen. Make
sure that you record the exact mass of sunscreen added in your notes. If your
sunscreen is too thick to drip, try moving a small amount into the beaker
with a stirring wand.
Add a few drops of water to the beaker and swirl/stir until
the mixture takes on a milky consistency. Try to break up the sunscreen blob
as much as possible.
Now, place the magnetic stirrer in the beaker and place the
beaker on the stirring plate. Slowly turn up the power on the stirrer. Now,
slowly add about 50 ml of isopropyl alcohol to the beaker. Allow this solution
to mix until most of the sunscreen flecks have disappeared.
Using the funnel, pour the beaker's contents into the volumetric
flask. Using the same funnel, add additional isopropyl alcohol to the flask
until the fluid's meniscus is just above the etched line in the glass (this
will mean that there is exactly 100 ml of solution present).
(2) Making Dilutions
Set up your two burettes on the burette stand. Note: the
burettes need to be closed (this means that the turning piece at the bottom
needs to be perpendicular to the glass tube).
Using the rinsed funnel, fill the burette on the right with
your remaining isopropyl alcohol.
Fill the burette on the left with your sunscreen solution.
Obtain 6 cuvettes. You may wish to label these with small
pieces of tape as A, B, C, D, E and "blank". Record the contents
of each in your notes.
Fill the "blank" cuvette with isopropyl alcohol.
Note: "filling" a cuvette really only requires that it be two thirds
full (or one third empty, for the pessimists out there).
Fill the cuvette labeled "E" with your sunscreen
solution. Set these two cuvettes aside.
You will now make a series of dilutions of your original
sunscreen solution. Make a table in your notes with three headings: "name",
"Parts Sunscreen Solution" and "Parts Isopropyl Alcohol,"
like the one below:
|
NAME
|
Parts Sunscreen Sol'n
|
Parts Isopropyl Alcohol
|
|
A
|
2
|
4
|
|
B
|
2
|
2
|
|
C
|
4
|
2
|
|
D
|
8
|
2
|
|
E
|
Pure
|
N/A
|
Use the burettes to drop 2 milliliters of sunscreen solution
and 2 milliliters of isopropyl alcohol into a beaker (note: "parts"
= "milliliters" in this lab). Swirl this slightly to stir and then
fill cuvette "A" with the solution. Empty the beaker and repeat
the process, using the parts listed for cuvettes B, C, and D.
If you would like to make additional dilutions in additional cuvettes, you
will improve your results and may be eligible for extra credit. Be sure to
record the name and the parts of each of the solutions you used.
(3) Scanning your cuvettes.
Follow the procedure that your teacher showed you for using
your spectrophotometer. Some basic guidelines will be discussed here.
Blank the spectrophotometer using the cuvette filled with
just isopropyl alcohol. This is the background for all of your sunscreen scans.
Scan each of your sunscreen cuvettes making sure that you
save each scan under a name you'll remember. For example, "Yourname_A.icn"
for cuvette "A" is much clearer than "171202_6.icn". When
you are finished using the machine, allow another group to scan their samples.
If you used a disk to store your scans, take it with you. Ask you teacher
about disposal instructions for your chemicals.
Analysis of Results
(1) Looking at the scans.
Open all of your sunscreen scans (A, B, C, D and E for most)
so that you can look at them together. If your program shows the graphs as
"wavelength vs. absorbance" you will be able to drag a pointer along
the screen. Wherever it intersects the graphs, it will display each of their
absorptions at the current wavelength.
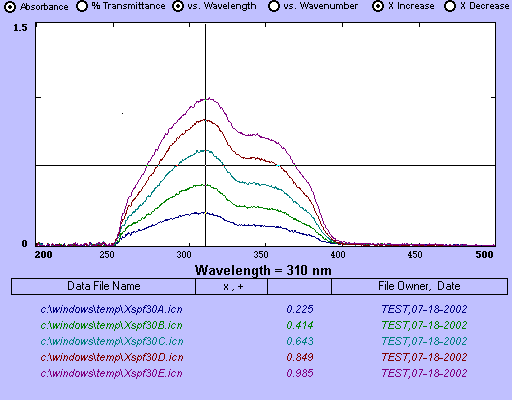
For Example:
The text at the bottom displays the file names that correspond
to the different sunscreen solutions and their respective absorptions at 310
nm. Note how the vertical line in the cross is also positioned at 310 nm,
thus selecting this wavelength.
Record the absorptions of the five solutions at their highest
point in your notes or data table. This need not be a sharp peak like the
one in the graph above, just the location of the curves' maximum values. Make
sure that you know which absorbance value corresponds to which concentration.
Note: although the curves have different concentrations, they will all peak
at about the same point.
(2) Doing the Math
It is now necessary to calculate the concentrations of each
of your sunscreen solutions in terms of milligrams of sunscreen per milliliter
of solution. Let's first figure out the concentration of your original solution
(sometimes called a "stock" solution). Take the number of grams
of sunscreen you used and multiply it by 1000 (this converts the grams into
milligrams). Now, since you made 100 ml of solution, divide the mass of the
sunscreen in milligrams by this number. For the purpose of explaining the
following procedure, this lab handout will use a stock concentration of 0.500
mg/ml (your value should be close, but probably won't be identical; anything
from 0.250 mg/ml to 0.750 mg/ml should work fine). You will need to refer
to the data table you made earlier. Use the following formula to calculate
the concentration of the different dilutions.
[(stock concentration) x (parts sunscreen)] ¸
[(parts sunscreen) + (parts isopropyl alcohol)]
Calculating the concentration of sample "A"
(0.500 mg/ml x 2) / (2 + 4) = (1.00 mg/ml)/(6)
= 0.167 mg/ml
Repeat this calculation for the other solutions you made.
Make another data table that compares the concentration of
samples A, B, C, D and E to their peak absorptions that you found earlier.
You will be asked to make a graph using these values later.
(3) Making a Beer's Law Plot
For more information on Beer's Law and making a Beer's Law
plot, CLICK HERE.
Beer's law demonstrates the direct relationship between concentration
and absorbance of solutions under ideal conditions. You will test this relationship
by graphing your concentration and absorbance data as points on a graph. The
concentration value will serve as the "x" and the absorbance will
serve as the "y" for your (x,y) data points. Do not worry about
putting units of absorbance on your graph, but do include all other necessary
graph features (title, labeled axes, etc.).
Draw a best-fit line through the data and calculate its slope.
Your teacher may allow you to use graphical analysis software to plot your
data. If so, use the "add trendline" feature to draw the line. Select
the "linear" option and display the equation of the line on your
graph.
VIEW LECTURE CONNECTIONS
(1) Do the raised portions of your scans correspond to the
UV region? What does this tell us about the sunscreens?
(2) Most sunscreens use separate chemicals to block different
regions of the UV wavelengths. Each chemical will provide its own absorbance
curve, and together these curves "meld" into the single curve produced
by your scan. Based on the bumps/peaks in your scans, how many active chemicals
would you say are contributing to the sunscreens absorbance?
(A) Extra Credit:
Color the regions that you think correspond to the different chemicals. Using
the active ingredients list on the back of your sunscreen, see if you can
identify which bump belongs to which chemical. You may need to do some research
to determine what parts of the UV Spectrum the chemicals block. It may also
be useful for you to know the breakdown of the UV range into its A, B and
C components. Your teacher may have some useful information about these investigations.
(3) What is the slope of your graph? What are the units of
the slope? What does this tell us? Compare your slope to the slope of another
lab group's graph, preferably one who used a different SPF sunscreen. Is there
any relationship between the slope and SPF?
(B) Extra Credit:
Make a graph of all the groups' slopes and SPF values. Use the various sunscreens'
SPF values as the "x" and the slope as the "y". Is there
a relationship between the two?
