Water-based Inks Lab -- Teacher Background
1 lab period for wet lab
1 class period for spectral analysis and preparation
Learning Goals
1. To compare and contrast two techniques in identifying components in an unknown solution
2. To use paper chromatography and UV-vis spectroscopy to identify the component colors of water-based inks in an aqueous (water) solution.
Background : Lecture Connections
Equipment/Materials: 12 pairs of students, divided between the two procedures
|
Chemicals
|
Equipment and Supplies
|
Instruments
|
| 7 colors of water-based markers--3-4 sets, including red, yellow, blue, green, orange, purple, black, and brown; other colors as available. | Chromatography paper* | UV-vis spectrometer |
| Water | 12--250-mL beaker | |
| 48 UV-vis cuvettes | ||
| 84 wooden splints | ||
| paper towel | ||
| pencil | ||
| 6 rulers | ||
| 6 scissors | ||
| tape | ||
| 12 -- 3.5" floppy disk for data storage |
* See Alternatives
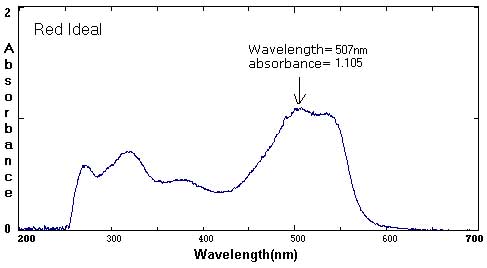
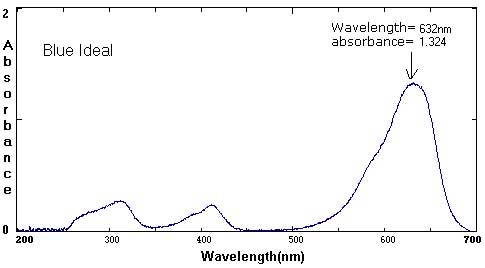
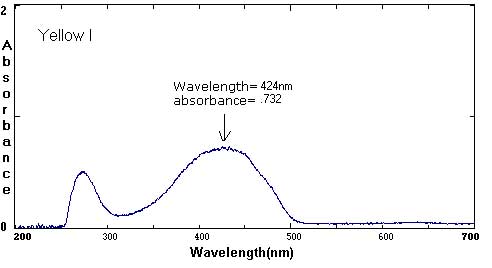
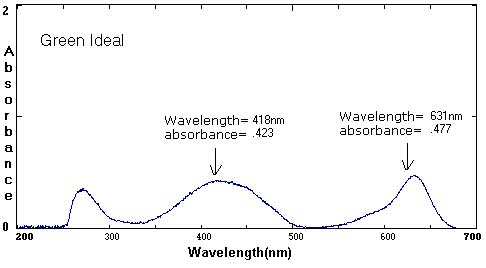
Sample Data: We have included sample ink spectra as a reference. Please keep in mind that different brands, different colors, and different techniques will affect the exact wavelength and absorbance values.




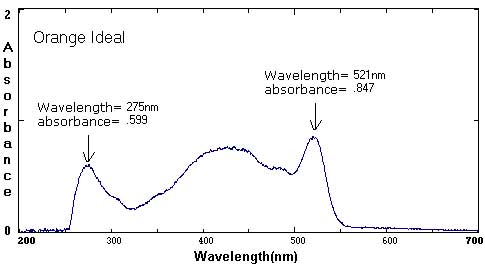
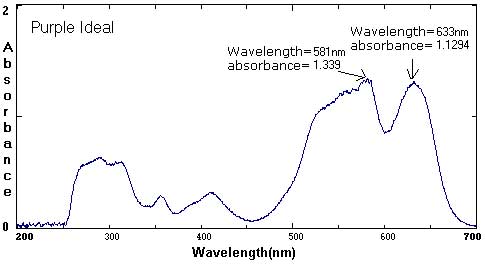
Although we focus on peaks within the visible light spectrum (between 400 nm-800 nm), peaks in the ultraviolet (UV) range (less than 400 NM) are also part of the unique 'fingerprint' of that color. For example, compare the UV peaks in the Green Ideal spectrum with that of the Yellow Ideal spectrum.
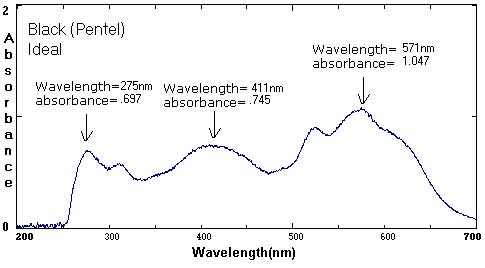
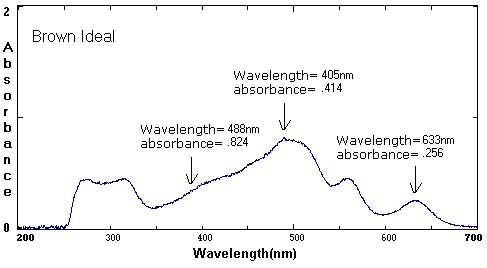
In these examples, the black and brown spectra are more complex mixes of colors than the secondary colors shown above. The black ink contains yellow, red, and blue pigments. Comparing the spectra with the chromatograms may help students identify peaks that are less obvious.
Safety: Students should follow general safety procedures regarding the handling of glassware.
Disposal: All materials are nontoxic. Although solutions may be flushed down the drain, it is good training for students to empty their cuvettes into labeled waste or used-materials containers. This procedure will encourage good disposal habits when more hazardous materials are used in the lab. Any unused chromatograms may be thrown in the trash.
Suggestions:
1. Have half the class begin with the paper chromatography while the other half starts with the UV-vis spectroscopy. The groups can switch procedures as they complete one part. This will make more efficient use of equipment and lab time.
2. Have a variety of water-based markers on hand. Sample results for this lab included inks from Crayola, Color Club, Pentel, Pilot, and LiquiMark (overhead markers). Children's washable markers are a good source of water-based inks in a variety of brands and colors.
3. As an extension, have students who used different brands of the same non-primary colors to compare their spectra and chromatograms. They may find that different brands have different compositions for the 'same' color, especially for the blank inks.
Troubleshooting: To correct poor spectra, try one or more of the following suggestions:
1. Blank the machine. Listen for the click that signals the blank has been read. Re-scan samples.
2. Be sure the cuvette is clean and dry, and inserted properly into the holder. (Moisture or fingerprints on the clear sides of the cuvette can interfere with the machine's reading.)
3. If the spectra are showing either jagged peaks or flattened plateaus instead of distinct, smooth peaks, the solution may be too concentrated. Dilute the solution and re-scan the sample.
4. You may also view the Spectra Library of Common Mistakes for further comparison.
Alternatives:
Maine State Learning Results (MSLR): This lab encourages skills listed in the following performance indicators under content area for secondary scores:
E. The Structure of Matter:
J. Inquiry and Problem Solving
Students will apply inquiry and problem-solving approaches in science and technology. Students will be able to:
- Make accurate observations using appropriate tools and units of measure.
- Verify , evaluate, and use results in a purposeful way. This includes analyzing and interpreting data, making predictions based on observed patterns, testing solutions against the original problem conditions, and formulating additional questions.
- Demonstrate the ability to use scientific inquiry and technological method with short-term and long-term investigations, recognizing that a there is more than one way to solve a problem. Demonstrate knowledge of different strategies.
K. Scientific Reasoning
Students will learn to formulate and justify ideas and to make informed decisions. Students will be able to:
3. Develop generalizations based on observations
4. Determine when there is a need to revise studies in order to improve their validity trough better sampling, controls, or data analysis techniques.
L. Communication
Students will communicate effectively in the application of science and technology. Students will be able to:
3. Make and use appropriate symbols, pictures, diagrams, scale drawings, and models to represent and simplify real-life situations and to solve problems.
4. Employ graphs, tables, and maps in making arguments and drawing conclusions.
7. Use computers to organize data, generate models, and do research for problem solving.